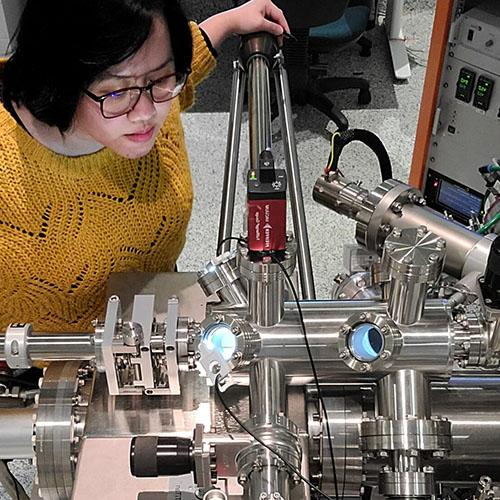
Australian Postgraduate Research Intern (APR.Intern) is Australia’s only national PhD and Masters by Research internship program spanning all sectors and disciplines.
APR.Intern is the industry engagement arm of the Australian Mathematical Sciences Institute (AMSI), and connects postgraduate research students with industry through short-term placements, empowering students to thrive in a practical research environment. For businesses, APR.Intern is a platform to access Australia’s brightest emerging research talent and accelerate innovation.
“APR.Intern is a fantastic program for R&D-heavy industries such as ours [medtech]. The support we received took the administrative load out of pursuing an academic research partnership and made it easy for all parties to achieve results”
Dr Andrew Sexton, Senior Mechanical Engineer at Inventia Life Science
© 2019 Australian Mathematical Sciences Institute | Website feedback: webmaster@amsi.org.au